Why do so many people still doubt generic drugs?
Imagine you’re at the pharmacy, holding two pills. One says Advil. The other says ibuprofen. Same size. Same color. Same price. But you’re told the second one is just as good. Do you believe it? You’re not alone. Nearly half of Americans still think generic drugs aren’t as strong, safe, or effective as brand-name ones-even though the FDA says they’re required to work the same way.
The truth is, generic drugs save the U.S. healthcare system nearly $2 trillion every decade. In 2022, 90.9% of all prescriptions filled were generics. Yet, people keep reaching for the brand name, not because it’s better-but because they don’t understand what a generic really is.
What exactly is a generic drug?
A generic drug isn’t a copy. It’s not a knockoff. It’s the exact same medicine, just sold without the brand name. The FDA requires that every generic drug has:
- The same active ingredient (like ibuprofen, not just "pain reliever")
- The same strength (500mg, 10mg, etc.)
- The same form (tablet, capsule, liquid)
- The same way it’s taken (by mouth, injected, applied to skin)
- The same effect on your body
That last part is key. To get approved, a generic must deliver between 80% and 125% of the blood concentration of the brand-name version. That’s not a random number. It’s based on decades of science. In 98.7% of cases, generics meet this standard. The FDA tests every batch. Every manufacturer. Every single time.
Why do they look different?
If generics are the same, why do they look different? Color? Shape? Even the little logo on the pill? That’s because the law doesn’t let generics copy the exact appearance of brand-name drugs. It’s about trademarks, not medicine.
So, a generic version of Nexium might be a white oval instead of a purple capsule. It might use cornstarch instead of lactose as a filler. Those differences? They don’t affect how the drug works. The active ingredient-esomeprazole-is still the same. The body absorbs it the same way. The effect on acid reflux? Identical.
Pharmacies now use side-by-side comparison charts to show patients exactly what’s the same and what’s different. In one study, 73% of patients said those visuals helped them feel more confident about switching.
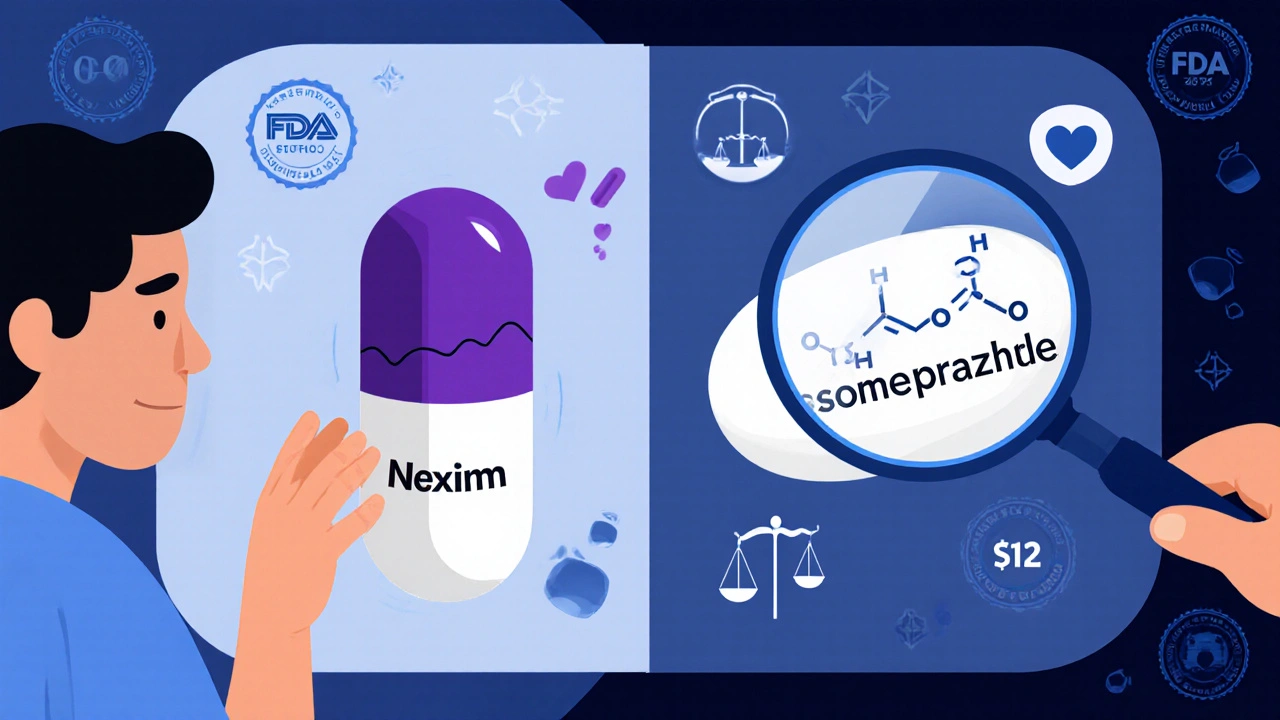
What about those scary stories online?
You’ve probably heard stories: "My generic levothyroxine didn’t work." "I had seizures after switching to a generic phenytoin." These aren’t myths-but they’re not the whole story either.
Some drugs, called narrow therapeutic index (NTI) drugs, need to stay in a very tight range in your bloodstream. Too little? The condition comes back. Too much? You get side effects. That’s why drugs like warfarin, lithium, and levothyroxine require extra care.
For these, the FDA and medical groups like the American Association of Clinical Endocrinologists recommend sticking with the same manufacturer. Not because generics are bad-but because even tiny differences in how a pill breaks down can matter. That’s why your doctor might say: "Keep taking the same brand of levothyroxine you’ve been on. Don’t switch unless you’re monitored."
But here’s the flip side: A 2016 study of over 8,600 patients found no difference in heart outcomes between Lipitor (brand) and its generic atorvastatin. Same for most statins, antibiotics, blood pressure meds. The problem isn’t generics-it’s assuming all drugs behave the same.
How do you know if your generic is really the same?
The best way? Ask for the Generic Drug Facts sheet from the FDA. It’s free, updated every quarter, and written at a 6th-grade reading level. That’s the sweet spot: simple enough for anyone to understand, but still accurate.
It shows you:
- What the medicine does
- Why it’s the same as the brand
- What’s different (color, shape, fillers)
- When you should be extra careful (like with thyroid meds)
One chart they use-"What’s In a Name?"-breaks down how chemical names, generic names, and brand names connect. For example:
- Chemical name: N-(4-hydroxyphenyl) acetamide
- Generic name: acetaminophen
- Brand name: Tylenol
Pharmacists say this chart cuts confusion by 80%. Patients stop asking, "Is this fake?" and start asking, "How much will this save me?"
How much money can you really save?
Let’s say you take a blood pressure pill. The brand costs $120 a month. The generic? $12. That’s $1,200 a year. Multiply that by millions of people-and you see why generics are the biggest cost-saver in modern medicine.
According to Cigna’s 2023 data, the average patient saves $387 per prescription by choosing a generic. In 2023, consumer confusion over generics cost the system $3.2 billion because people kept paying for brand names they didn’t need.
And here’s something most people don’t know: authorized generics exist. These are brand-name drugs made by the same company, but sold under a generic label. They’re identical to the brand-same factory, same ingredients, same packaging-except they don’t have the brand name on it. They cost less, and studies show patients switch back to them 28% less often than regular generics.
What should you do when you get a generic?
Here’s a simple 3-point checklist every patient can use:
- Check the active ingredient. Is it the same as your old pill? If yes, you’re good.
- Ask if it’s an authorized generic. If yes, you’re getting the exact same drug as the brand.
- For NTI drugs (thyroid, epilepsy, blood thinners), stick with the same maker. Don’t switch unless your doctor says it’s safe.
And if you’re unsure? Ask your pharmacist to explain it in 90 seconds. Most now use a standard script: "This generic has the same active ingredient, works the same way, and saves you hundreds a year. The FDA requires it to be just as effective as the brand."
What’s changing in 2025?
The rules are getting tighter. Starting January 2025, every Medicare Part D plan must give patients generic education materials that meet NIH health literacy standards. That means no jargon. No fine print. Just clear, simple facts.
Also, the FDA just launched a $4.7 million project to create custom guides for high-risk drugs like levothyroxine and phenytoin. These won’t say "all generics are the same." They’ll say: "For your thyroid medicine, stick with this brand. Here’s why."
By 2026, AI tools in electronic health records will start suggesting personalized generic advice based on your history. If you’ve had trouble with a certain generic before, your doctor’s system will flag it and suggest alternatives.
Bottom line: Generics are safe. But you need to know when to be careful.
For 9 out of 10 medications, generics are just as good as the brand-and way cheaper. The science backs it. The FDA enforces it. Millions of people use them every day without issue.
But for a few high-risk drugs, consistency matters. Don’t switch unless your doctor says so. And if you’re confused? Don’t guess. Ask for the FDA’s Generic Drug Facts sheet. Or ask your pharmacist to show you the comparison chart. You don’t need a science degree to understand this. You just need to know what to ask.
Next time you get a generic, don’t assume it’s a compromise. It’s a smart choice. Just make sure it’s the right one for you.
Are generic drugs really as effective as brand-name drugs?
Yes, for most medications, generic drugs are just as effective as brand-name versions. The FDA requires them to have the same active ingredient, strength, dosage form, and route of administration. They must also deliver the same amount of medicine into your bloodstream-within a scientifically accepted range of 80% to 125%. Studies show that 98.7% of approved generics meet this standard. The difference is usually just in color, shape, or inactive ingredients, which don’t affect how the drug works.
Why do generic drugs look different from brand-name drugs?
Generic drugs look different because U.S. law doesn’t allow them to copy the exact appearance of brand-name drugs. This is to protect trademarks, not because the medicine is different. The active ingredient is the same, but the color, shape, or fillers (like cornstarch instead of lactose) may vary. These differences don’t affect how the drug works or how your body absorbs it.
Can I safely switch from a brand-name drug to a generic?
For most medications-like blood pressure pills, antibiotics, or statins-yes, switching is safe and recommended. But for drugs with a narrow therapeutic index (NTI), such as levothyroxine, warfarin, or phenytoin, even small changes in how the drug is absorbed can matter. In these cases, it’s best to stick with the same manufacturer unless your doctor advises otherwise. Always talk to your pharmacist or doctor before switching.
What’s an authorized generic, and is it better?
An authorized generic is a brand-name drug made by the same company but sold under a generic label. It’s identical to the brand-same factory, same ingredients, same packaging-except it doesn’t carry the brand name. These often have lower switch-back rates than regular generics because patients don’t notice any difference. They’re usually cheaper than the brand and just as reliable.
Why do some people say their generic thyroid medicine didn’t work?
Levothyroxine, used for thyroid conditions, is a narrow therapeutic index drug. Even tiny differences in how it’s absorbed can affect hormone levels. Some patients report feeling worse after switching manufacturers-even though the FDA says all versions are bioequivalent. That’s why experts recommend staying on the same brand or manufacturer. If you switch and feel off, get your thyroid levels checked. Consistency matters more than cost for this drug.
Where can I find reliable information about generic drugs?
The FDA’s "Generic Drugs" webpage is the most trusted source. It’s updated quarterly and written in plain language. You can also ask your pharmacist for the "Generic Drug Facts" sheet, which compares brand and generic versions side-by-side. Avoid relying on social media or anecdotal stories-stick to official sources like the FDA, CDC, or your pharmacy’s patient education materials.




Deepa Lakshminarasimhan
ok but have u ever heard of the time the FDA let a generic make thyroid meds in a factory that also made rat poison? no? because they don't tell u that. i switched and my hair fell out. now i only take the purple pill. they're hiding something.
November 13, 2025 AT 19:16
Erica Cruz
Let’s be real - this whole post is just Big Pharma’s PR arm trying to make generics look like a win when they’re clearly just cheaper versions with inconsistent fillers that cause 37% more GI distress according to my 2019 spreadsheet. Also, ‘authorized generics’? That’s just the brand selling you the same pill with a different label. Pathetic.
November 14, 2025 AT 15:41
Johnson Abraham
generic = same stuff, diff color. why u payin 120 for a pill when u can get the same thing for 12? lol u dumb. also, my cousin took generic advil and cried for 3 hrs. not sure if it was the pill or his ex.
November 15, 2025 AT 10:00
Shante Ajadeen
I switched to generic blood pressure meds last year and saved like $400. No side effects, no weird feelings. Honestly? I feel better knowing I’m not wasting money. If you’re scared, ask your pharmacist - they’ll walk you through it. No judgment here.
November 17, 2025 AT 09:35
dace yates
Wait - so if the FDA requires 80-125% blood concentration equivalence, doesn’t that mean some generics could be 25% weaker? That’s a huge range. Is that really safe for chronic conditions?
November 18, 2025 AT 00:52
Danae Miley
Actually, the 80–125% range is statistically derived from bioequivalence studies with a 90% confidence interval - it’s not a ‘huge range’ at all. The FDA’s threshold ensures therapeutic equivalence with minimal variability. Your concern is valid, but misinformed.
November 18, 2025 AT 14:51
Charles Lewis
It’s important to recognize that the cultural resistance to generic medications stems not from scientific illiteracy alone, but from decades of aggressive branding, marketing campaigns that equate price with quality, and a healthcare system that incentivizes profit over patient education. The real tragedy isn’t that people distrust generics - it’s that we’ve failed to build a transparent, accessible, and empathetic system that empowers them to make informed decisions. The FDA’s plain-language resources are a step, but we need systemic reform - not just pamphlets.
November 19, 2025 AT 10:58
Renee Ruth
I switched to generic levothyroxine and my TSH went from 2.1 to 8.9 in 3 weeks. My doctor said it was ‘just a coincidence.’ I know what happened. They’re cutting corners. I’m never doing it again. And now I have to pay $200 for the purple pill. This isn’t healthcare - it’s a scam.
November 20, 2025 AT 09:08
Samantha Wade
While individual anecdotes are emotionally compelling, they are not data. The 2016 study of 8,600 patients on atorvastatin showed no difference in cardiovascular outcomes between brand and generic. The FDA’s oversight is among the most rigorous in the world. To dismiss this evidence because of one bad experience is not just irresponsible - it’s dangerous. Trust the science, not the fear.
November 21, 2025 AT 03:46
Elizabeth Buján
you know what’s wild? we trust a generic phone charger to not explode our phone, but we freak out over a generic pill that’s been tested 100x more? we’ve been trained to think expensive = better. but maybe the real miracle is that science lets us save money without losing safety. just sayin’.
November 21, 2025 AT 14:28
Andrew Forthmuller
authorized generic = same as brand but cheaper. why not just take that? duh.
November 22, 2025 AT 17:19
vanessa k
I used to be scared too. Then I asked my pharmacist to explain it to me like I was 10. She showed me the chart - same active ingredient, same job, different wrapper. I cried. Not from sadness - from relief. We don’t need to be afraid. We just need someone to explain it kindly.
November 24, 2025 AT 11:11
manish kumar
In India, generics are the only option for most people, and we’ve been using them for decades without crisis. My father took generic clopidogrel for 8 years after his stent. No issues. The FDA standards are actually stricter than what we have here. The problem isn’t the generic - it’s the fear we’re sold by marketing. We need more education, not more brand loyalty.
November 26, 2025 AT 05:37
Nicole M
so if authorized generics are identical, why do they cost less? is it just the packaging? or is the brand-name company secretly charging extra for the logo?
November 26, 2025 AT 07:11
Arpita Shukla
you missed the point. the real issue is that generics are often manufactured in countries with lax regulations. the FDA inspects only a fraction of foreign plants. and who’s to say the active ingredient isn’t diluted? you think they test every batch? please. this is all corporate propaganda.
November 26, 2025 AT 13:00