You’ve been on the same medication for years. Your doctor switches you to the generic version. Suddenly, you feel different. Maybe your anxiety spikes. Maybe your blood pressure feels off. You think: generic drugs don’t work as well. But here’s the truth-your body isn’t reacting to a weaker drug. It’s reacting to your mind.
They’re the Same Medicine, But Your Brain Says Otherwise
Generic drugs contain the exact same active ingredient as their brand-name counterparts. Same dose. Same shape. Same way your body absorbs it. The U.S. Food and Drug Administration (FDA) requires generics to be bioequivalent-meaning they deliver the same amount of medicine into your bloodstream within the same time frame as the brand-name version. The allowed variation? Between 80% and 125%. That’s not a loophole. That’s science. For nearly all medications, this tiny range has zero clinical impact. Yet, 13% of Americans still believe brand-name drugs are more effective. Even more-20%-think generics cause more side effects. Why? Because perception doesn’t care about data. Think about it: you’ve seen the flashy ads for brand-name drugs. You know the name. You’ve heard it from your neighbor, your cousin, your aunt. You trust that name. The generic? It’s a plain white pill with a code on it. No logo. No story. It looks like something you’d get at a discount store. Your brain connects appearance with quality. And suddenly, even though the chemistry is identical, your body starts to feel worse.The Nocebo Effect: When Expectations Make You Sick
There’s a name for this: the nocebo effect. It’s the evil twin of the placebo effect. Where placebo makes you feel better because you believe a treatment works, nocebo makes you feel worse because you believe it won’t. A 2023 study in JAMA Network Open proved this. Patients told their new generic pill was “just as effective” as the brand had 34% better adherence. Those told it was “less effective” had 41% worse outcomes-even though the pill was identical in both groups. The drug didn’t change. The belief did. Pharmacists in Perth, Sydney, and rural Alabama report the same thing: patients who’ve been stable on a brand-name drug for years suddenly report side effects after switching to generic. Their labs show no change. Their symptoms? They’re real. But they’re not caused by the medicine. They’re caused by fear.Why Some Groups Are More Skeptical
This isn’t random. The perception gap is wider among certain groups. Non-Caucasian patients are nearly twice as likely to doubt generics. Rural communities are more likely to believe generics are “for poor people” or “not real medicine.” These aren’t just myths. They’re rooted in history. In the U.S., low-income patients have long been steered toward generics-not because they’re inferior, but because they’re cheaper. Over time, that message got twisted. “They gave me the cheap one” became “They gave me the bad one.” A 2015 study in the Journal of General Internal Medicine found 43% of non-Caucasian patients expressed skepticism about generic equivalence, compared to 29% of Caucasian patients. And 56% of non-Caucasian patients asked for brand-name drugs-even when they couldn’t afford them. It’s not about intelligence. It’s about trust. If you’ve been let down by the system before-if you’ve been prescribed something that didn’t work, or if you’ve seen loved ones struggle with healthcare costs-you’re more likely to question what’s being handed to you.
Manufacturing Myths: Are Generics Made in Worse Facilities?
You’ve heard it: “Generics are made in China. Brand drugs are made in the U.S.” The truth? The FDA inspects both brand and generic manufacturing sites using the same standards. In fact, the same factories often make both. Many brand-name companies produce their own generics after the patent expires. The difference? The label. A 2016 FDA report did find that foreign generic facilities received more inspectional observations than U.S. ones. But here’s the catch: those observations were mostly paperwork issues-documentation, labeling, minor recordkeeping-not safety or quality problems. The final product? Still meets the same standard. If a generic pill fails to meet FDA requirements, it doesn’t hit the shelf. Not ever. Not even once. That’s not a rumor. That’s policy.The Real Cost of the Perception Gap
Generics save Americans $1.7 trillion between 2009 and 2019. That’s billions in out-of-pocket savings for families, lower premiums for employers, and less strain on Medicare and Medicaid. But when people refuse generics-or stop taking them altogether-the cost isn’t just financial. It’s health. A 2019 study found that 22% of patients who believed generics were inferior stopped taking their medication. That’s more than double the rate of those who didn’t hold that belief. For someone with high blood pressure, diabetes, or depression, that’s not a minor setback. It’s a medical crisis. One pharmacist in Texas shared a story: a woman on levothyroxine for hypothyroidism switched to generic. She felt tired. She thought it wasn’t working. She stopped taking it. Three months later, she was hospitalized with severe heart complications. Her TSH levels? Off the charts. The drug was fine. The belief wasn’t.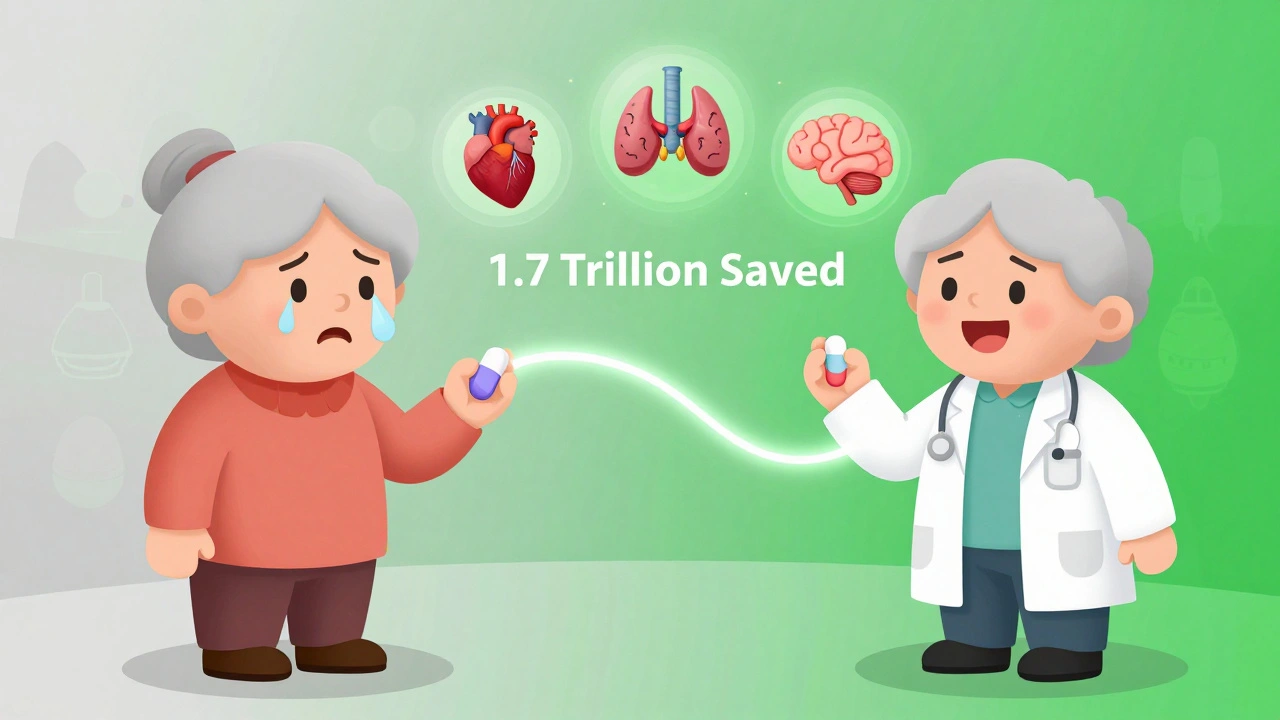
What Works: How to Bridge the Gap
So how do you fix this? It’s not about shouting statistics. It’s about communication. Doctors and pharmacists who show patients the exact same active ingredient on both the brand and generic labels? They see 87% more patients accept the switch. Showing FDA documentation? That improves acceptance by 76%. Talking directly about the nocebo effect? That helps 68% of patients. The FDA’s “It’s the Same Medicine” campaign reached 27 million people. But only 19% remembered it. Why? Because it was too clinical. Too dry. Too far removed from real lives. What works better? Simple language. Real stories. Visuals. A pharmacist saying: “This pill has the same active ingredient as your old one. The only difference? It costs $15 instead of $120. And it’s been taken by over 10 million people without issue.”What’s Changing-and What’s Not
The FDA is finally taking this seriously. In 2023, they started requiring therapeutic equivalence ratings on generic packaging. That’s a small step-but it’s a start. In 2024, they’re launching “Equivalence Explorer,” an online tool that lets you compare brand and generic drugs side by side. The American Medical Association now requires doctors to get training on how to talk about generics. That’s huge. Because the person who prescribes the drug has more influence on patient trust than any ad or website. But here’s the hard truth: perception doesn’t change fast. A 2023 analysis predicts it will take over 12 years for skepticism among non-Caucasian patients to match levels among Caucasian patients-at current rates. And brand-name companies? They’re still spending $1.8 billion a year on marketing that makes generics seem less trustworthy. Not by lying. But by implying. By using phrases like “clinically proven” or “trusted by millions” on their ads-while generics can’t say the same.You’re Not Crazy. But You Might Be Misinformed
If you’ve switched to a generic and felt worse, you’re not imagining it. Your symptoms are real. But they’re likely not caused by the drug. They’re caused by the story you’ve been told. The science is clear: generics work. They’re safe. They’re identical in active ingredients. They’re held to the same standards. The only thing different? The price. And the packaging. Before you assume the generic isn’t working, ask: Did I get the same active ingredient? Did my doctor explain why the switch happened? Did I hear someone say, “Generics are cheaper, so they’re not as good”? That’s the real problem. Your health matters. So does your peace of mind. Don’t let a label make you feel like you’re settling for less. You’re not. You’re getting the same medicine. Just without the marketing.Are generic drugs really as effective as brand-name drugs?
Yes. Generic drugs must contain the exact same active ingredient, strength, dosage form, and route of administration as the brand-name version. The FDA requires them to prove bioequivalence-meaning they deliver the same amount of medicine into your bloodstream at the same rate. For over 90% of medications, this means they work identically.
Why do some people feel worse after switching to a generic?
This is often due to the nocebo effect-when expecting a negative outcome causes real physical symptoms. If you believe the generic is inferior, your brain can trigger side effects like fatigue, anxiety, or headaches-even if the drug is chemically identical. Studies show patients told generics are “just as effective” have better outcomes than those told they’re “less effective.”
Are generic drugs made in lower-quality facilities?
No. All drug manufacturing facilities-whether making brand-name or generic drugs-must meet the same FDA standards for Good Manufacturing Practices (cGMP). Many brand-name companies even produce their own generics after patents expire. The FDA inspects both types of facilities using the same criteria.
Can generics cause more side effects than brand-name drugs?
Not because of the drug itself. The active ingredient is identical. Any difference in side effects is likely due to inactive ingredients (like fillers or dyes), which can vary slightly. But these are strictly regulated and rarely cause issues. If you experience new side effects after switching, talk to your doctor-but don’t assume it’s the generic’s fault.
Why do doctors still prescribe brand-name drugs sometimes?
Most of the time, they don’t. Generics make up 90% of prescriptions in the U.S. But in rare cases-like with narrow therapeutic index drugs (e.g., warfarin, levothyroxine)-some doctors prefer to stick with a brand they’ve seen work consistently for a patient. This is usually based on individual history, not a belief that generics are inferior. In most cases, switching is safe and effective.
How can I be sure my generic is safe?
Check the active ingredient on the label. It should match your brand-name drug exactly. Ask your pharmacist to show you the FDA’s Therapeutic Equivalence Rating (usually printed on the bottle). You can also visit the FDA’s website and search your drug by name-both brand and generic versions will appear with matching approval details. If it’s approved by the FDA, it’s safe.




val kendra
Look, I get it. I switched to generic levothyroxine last year and felt like garbage for two weeks. Turned out I was stressing so hard about it that my cortisol spiked. My doc showed me the FDA sheet - same active ingredient, same bioavailability. I stopped doomscrolling and started taking it like a pill, not a gamble. My energy came back. It’s not magic. It’s math.
December 4, 2025 AT 12:31
Karl Barrett
The nocebo effect is a fascinating neurophenomenon rooted in predictive coding theory - your anterior cingulate and insular cortex are essentially generating somatic symptoms based on top-down expectations. The pharmacokinetic equivalence is statistically non-inferior, but the psychosocial construct of ‘brand = trust’ overrides biopharmaceutical reality. We’re not just treating disease; we’re managing belief systems encoded in neural pathways.
December 5, 2025 AT 07:21
Jake Deeds
Oh please. You’re telling me my grandma’s 80-year-old heart doesn’t know the difference between a pill with a logo and one that looks like it was printed on a Staples printer? I’ve seen people crash because they were switched. It’s not ‘in their head’ - it’s their body remembering what it’s used to. You sound like a pharma rep with a PowerPoint.
December 5, 2025 AT 10:13
Ben Choy
My mate in Manchester had the exact same thing with his blood pressure med. Switched to generic, felt dizzy. Turned out he was so convinced it wouldn’t work he stopped sleeping. Doc gave him the same pill in the brand box. He was fine. Mind’s a weird thing 😅
December 5, 2025 AT 22:21
Elizabeth Crutchfield
i switched to generic adderall and felt like a zombie for a week. then i realized i was just scared. now i take it like a champ. its the same damn pill. why do we make this so hard??
December 7, 2025 AT 13:15
Rachel Bonaparte
Let’s be real - most generics are made in China by factories that get inspected once every 5 years. The FDA is corrupt. They let stuff through because Big Pharma owns them. You think they’d let a pill with 80% bioavailability be sold to the public if they weren’t getting kickbacks? Wake up. This isn’t science - it’s a scam to make poor people sick so they buy more drugs later.
December 7, 2025 AT 17:40
Scott van Haastrecht
Oh wow. Another ‘it’s all in your head’ article from someone who’s never had to take a pill that made them feel like they’re dying. You know what’s real? When your heart races, your hands shake, and your doctor shrugs and says ‘it’s the nocebo effect.’ Meanwhile, your insurance company saved $80. You’re not a patient. You’re a cost center.
December 8, 2025 AT 21:53
George Graham
I work in a rural clinic. A lot of folks here grew up thinking ‘cheap medicine = bad medicine.’ We started showing them the FDA labels side by side - same active ingredient, same expiration date, same manufacturer sometimes. One woman cried because she realized she’d been avoiding her meds for years out of shame. It’s not about facts. It’s about dignity.
December 10, 2025 AT 13:14
Ollie Newland
There’s a reason why warfarin’s a special case. Therapeutic window’s razor-thin. Even 5% variation can throw INR off. I’ve seen patients stabilize on brand, then crash on generic. It’s not placebo - it’s pharmacokinetics. Don’t generalize. Some drugs? Stick with the brand. No shame in that.
December 10, 2025 AT 16:15
Rebecca Braatz
If you’re scared to switch, talk to your pharmacist. Bring your old bottle. Ask them to show you the active ingredient. Write it down. Say it out loud. You’re not weak for doubting - you’re human. But you’re also powerful enough to rewire that fear. One pill at a time.
December 11, 2025 AT 16:29
Michael Feldstein
What if we just… stopped calling them ‘generics’? What if we called them ‘equivalent medications’? The word ‘generic’ sounds like ‘basic’ or ‘boring’ - like it’s not real. But it’s the same damn chemistry. Maybe the language is part of the problem.
December 13, 2025 AT 15:45
jagdish kumar
Reality is a mirror. You see what you believe. The pill is the same. The fear is the drug.
December 13, 2025 AT 18:37
Pavan Kankala
They say generics are safe. But who’s really checking? The same people who said asbestos was fine. The same people who said cigarettes were harmless. Trust the system? Nah. I take the brand. Even if it costs my rent.
December 14, 2025 AT 11:19